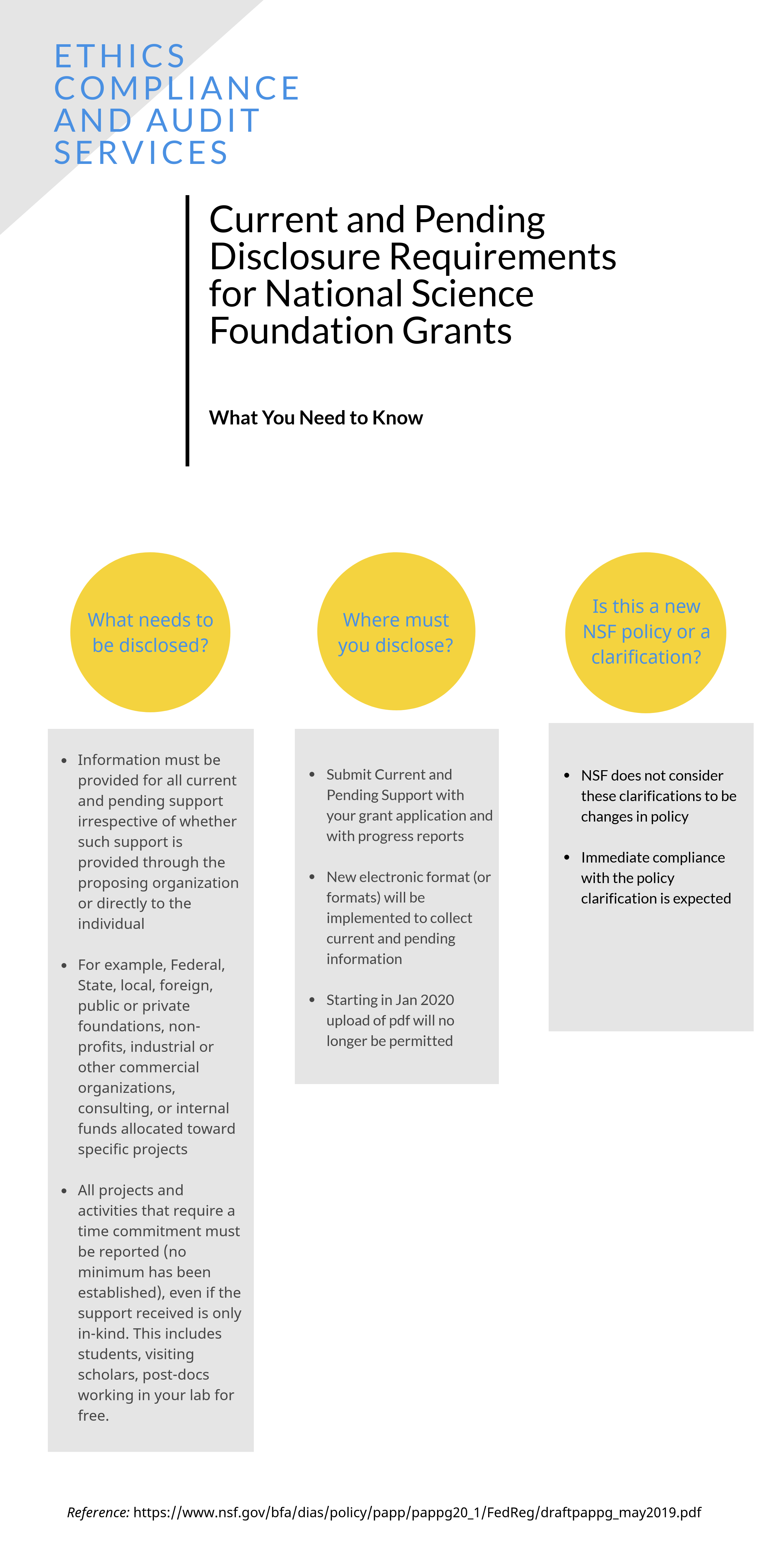
|
Who funds your current research? Make sure to let NIH know. It is required.
Institutions and investigators must disclose all forms of what is termed “other support” when
applying for and receiving NIH grants. Other support, as described in the NIH Grants Policy Statement (GPS)
Section 2.5.1, includes all resources, regardless of whether or not they have monetary value, available
in direct support of an individual's research endeavors.
This is not new, but rather a long-standing requirement for those seeking NIH grants to be fully transparent regarding all of their research activities both domestic and foreign, which is critical for prudent fiscal management, accountability, and stewardship of U.S. taxpayer funds.
So, do you need to report those other NIH grants you have? Yes. What about a contract from another federal agency? Yes. Grants or contracts that go through another institution, including institutions in foreign countries? Yes. Commercial funds? Yes. Domestic or international positions held by senior/key personnel? Yes. In kind lab or office space? Yes. Scientific materials? Yes. Even if it has no monetary value? Yes. Affiliations (even if described as honorary or adjunct) with foreign entities or governments, including talents programs? Yes.
NIH uses this information to ensure that all resources made available to an investigator, including any foreign activities, are considered prior to making an award. With this in hand, we will know that sufficient levels of effort are committed to the project, there is no scientific, budgetary, or commitment overlap, and only the funds necessary to the approved project are included in the grant award.
We recently published a Guide Notice to clarify what is meant by foreign activities as they relate to other support and what NIH expects to be disclosed. As part of this notice, applicants are reminded they must also promptly notify NIH if previously submitted just-in-time information is substantively changed prior to award or at the time of the progress report, which could lead to budgetary, scientific, or commitment overlap.
Since institutions are the applicants for and recipients of NIH funding, they are responsible for ensuring that all materials submitted to NIH are complete and accurate. This means they must also ensure individual investigators make all appropriate disclosures to them regarding other support, affiliations, and financial interests. Sometimes we discover potential issues involving institutions not accurately reporting other support, including foreign support through foreign institutions, associated with their NIH award. Sometimes it’s because investigators don’t report their foreign research activities to their American institutions. In such cases, we can take (and have taken) action. Depending on the severity and duration of the noncompliance (see NIH GPS Section 8.5), we may contact the affected institutions, impose specific award conditions, disallow costs, withhold future awards for the project or program, suspend the award activities, make a referral for investigator suspension or debarment, or terminate the award.
I want to be clear that we are focusing our efforts on enhancing research integrity across all our processes and systems. The extraordinary contributions of foreign nationals to American science are indisputable. As just one example, 24% of U.S. Nobel prizes have been awarded to foreign-born scientists. The biomedical research workforce continues to be greatly enriched and strengthened by scientists who come to our shores from many parts of the world. The overwhelming majority of researchers participating in NIH grants, whether U.S.
or foreign-born, are honest contributors to the advancement of knowledge that benefits us all. Driving away talented scientists from other countries would have a profoundly negative effect on American productivity.
We appreciate the efforts of recipient organizations to partner with us to improve reporting of all sources of research support and international collaborative research. These obligations are instrumental to protecting the integrity of biomedical research. Working together, we can be better assured that federal funding decisions are sound, proprietary information is protected, and compliance with grant terms is achieved.
If you have questions, please refer to the FAQs, the Guide Notice, or send an email to grantscompliance@od.nih.gov for additional assistance.
Mike Lauer | July 11, 2019 at 7:39 am | Tags: foreign, other support | Categories: Policy | URL: https://wp.me/p7Dr3j-4WK
|